Medical Device and In-Virto Diagnostics (IVD):
The global regulatory landscape for medical devices and IVDs is challenging and complex. At PharBioMed, we offer regulatory consulting services to support every stage of the medical device/IVD product lifecycle. Our global team of regulatory experts develops optimal regulatory strategies tailored to your organization's device and target markets. Our scientific and clinical writing team is equipped to handle all your medical device submission requirements. With our in-depth knowledge of regulations and processes, we expedite the medical device registration and submission process while maintaining the highest quality standards.
Let us assist you with:
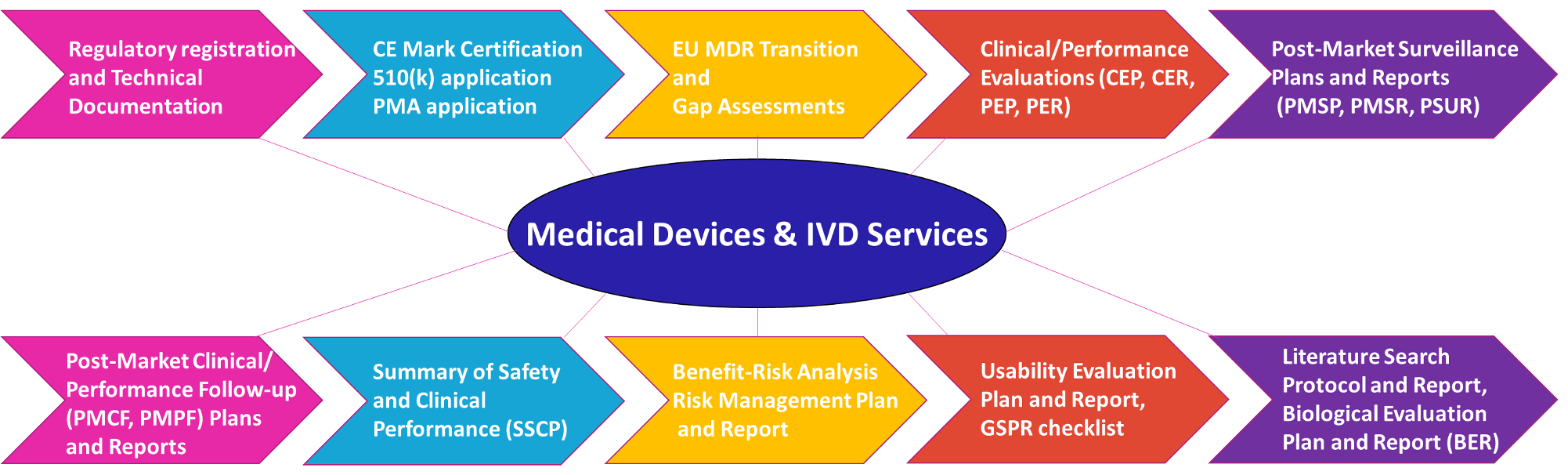
Medical Device/IVDR Registration Regulatory Affairs:
- Establishing Regulatory Pathways and Registrations
- Technical Documentation and Obtaining CE Mark
- MDR/IVDR Transition and Gap Analysis
- Design Dossiers
- Pre-Market Approval (PMA) Application
- 510(k) Submission
- Device Classification
- Summary Technical Documentation (STED)
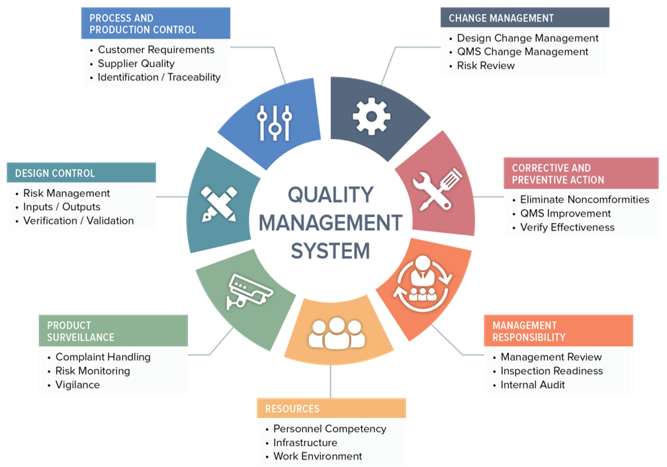
Medical Device/IVDR Quality Assurance:
- Establishment of ISO 13485 Quality Management System (QMS)
- Implementation of Unique Device Identification (UDI) and EUDAMED
- Readiness for MDSAP - Medical Device Single Audit Program
- Support for Internal and External Audits
- GSPR Checklist
Medical Device/IVDR Clinical Affairs:
- Clinical/Performance Evaluation Strategy
- Clinical/Performance Evaluation Plans and Reports (CEP, CER, PEP, PER)
- Literature Search Protocol and Report for Devices Under Evaluation and State-of-the-Art
Medical Device/IVDR Safety Data Management:
- Complaint Handling and Adverse Event Reporting
- CAPA and FSCA Reporting
- Safety Signal Management
- Literature Monitoring
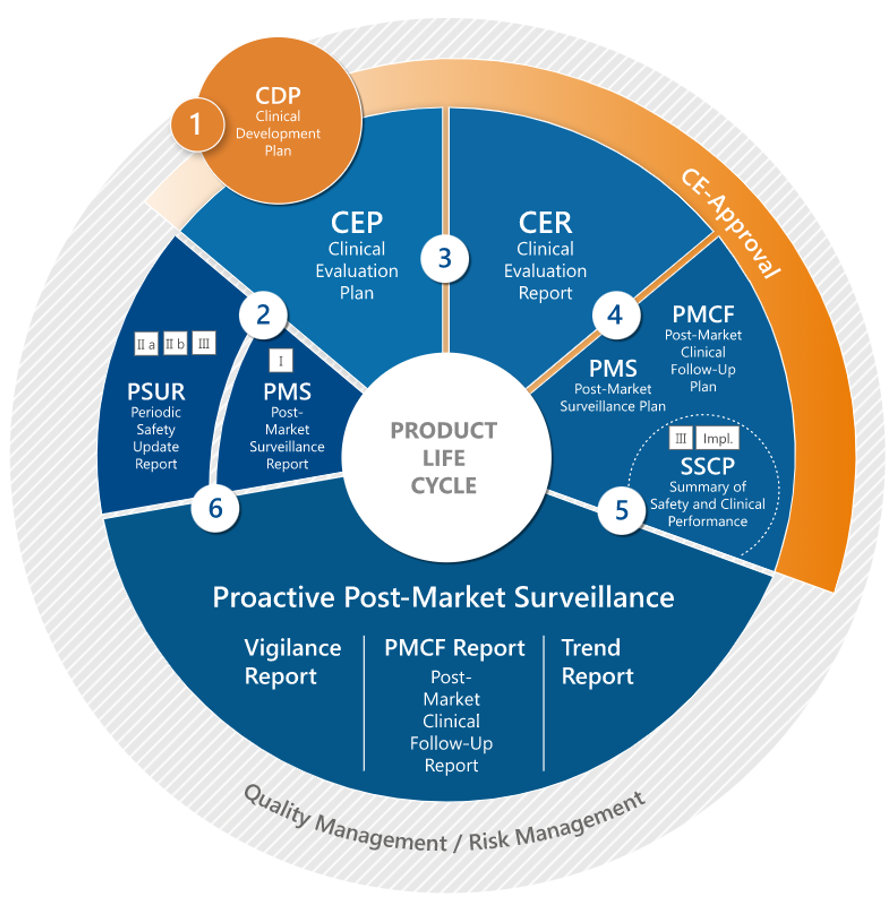
Medical Device/IVDR Post-Marketing Surveillance and Risk management:
- Post-Market Surveillance Plans and Reports (PMSP, PMSR)
- Periodic Safety Update Reports (PSURs)
- Strategies for Generating and Collecting PMCF Data
- Post-Market Clinical/Performance Follow-up Plans and Reports (PMCF, PMPF)
- Summary of Safety and Clinical Performance (SSCP)
- Benefit-Risk Analysis, Risk Management Plan, and Report
Medical Device Usability and Biological Evaluation:
- Usability Evaluation Plan and Report
- Biological Evaluation Plan and Report (BER)